Lutetium‑177 PSMA Therapy in Germany
Lu‑177 PSMA therapy—often called Lutetium‑177 PSMA‑617 or by its brand name Pluvicto—is transforming the way we treat advanced prostate cancer. Unlike conventional therapies that target the whole body, this innovative treatment delivers radiation directly to cancer cells, minimizing harm to healthy tissues. It’s a shining example of precision medicine—where science meets compassion.
Germany stands at the forefront of this medical breakthrough, having played a key role in developing and refining Lu‑177 PSMA therapy. Since one size doesn’t fit all, many of the country’s leading cancer centers address the challenges of metastatic cancer by offering this cutting-edge therapy as part of a personalized treatment plan.
Lu-177 PSMA therapy is typically administered by multidisciplinary team of healthcare experts, including:
- Nuclear medicine specialists
- Urologists
- Radiation oncologists
- Medical Oncologists
- Medical Physicists
- Other healthcare professionals and support staff, including nurses and technicians
Their collaborative approach ensures personalized treatment plans for each patient based on PSMA PET scans, overall health, prior treatment history, and specific needs of patients traveling from other countries.
If you or your loved one is seeking metastatic prostate cancer treatment through Lu-177 PSMA therapy in Germany, the Cancer Rounds team can provide complete assistance—serving as your anchor in a foreign healthcare system by guiding you through appointments, simplifying decisions, and ensuring a smooth treatment journey.
What is Lutetium‑177 PSMA Therapy & How It Works?
Lu‑177 PSMA is a targeted radioligand therapy, considered among the best treatments for metastatic castration-resistant prostate cancer (mCRPC). It is a remarkable connection of nuclear science and precision oncology. It harnesses the power of a radioactive isotope, Lutetium‑177, and couples it with a small molecule (the ligand, such as PSMA-617) designed to seek out and attach to Prostate-Specific Membrane Antigen (PSMA)—a
Once this radioligand is injected into the bloodstream, it begins its precise journey. Like a guided missile, it homes in only on the cells expressing PSMA, binding tightly to them. What makes this therapy so revolutionary is what happens next: Lutetium‑177 emits beta radiation right at the cancer cell, causing internal damage that leads to cell death—minimising damage to healthy tissues. This means fewer systemic side effects and a therapy tailored to attack where it’s needed most.
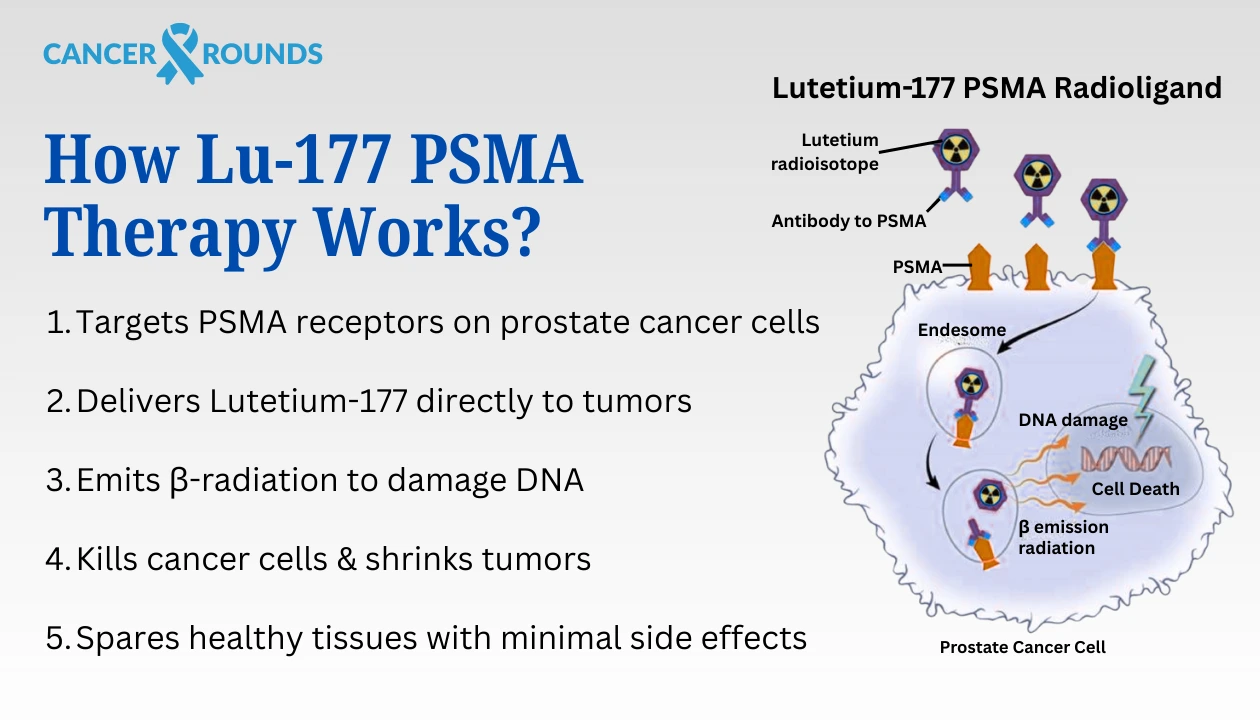
This targeted mechanism has made Lu‑177 PSMA Therapy for Hormone-Resistant Prostate Cancer in Germany one of the most promising alternatives for those with limited treatment options. In fact, it’s increasingly seen as a vital alternative for patients who’ve exhausted chemotherapy or androgen deprivation therapy.
Types of Prostate Cancer That May Benefit
Lu‑177 PSMA therapy is most commonly used for:
- Metastatic Castration-Resistant Prostate Cancer (mCRPC) – cancer that has spread beyond the prostate and is no longer responding to hormonal therapy.
- Advanced Hormone-Sensitive Prostate Cancer – being studied for earlier stages of aggressive disease.
- Biochemically Recurrent Prostate Cancer – in some trials, Lu‑177 PSMA is being explored for early relapse where PSMA uptake is high.
This makes Lu‑177 PSMA Therapy role in early prostate cancer cases in Germany a promising area of research and clinical trials.
How Is It Diagnosed and Matched to the Patient?
Before receiving Lu‑177 PSMA therapy, patients undergo a special imaging test called a PSMA PET scan (usually with Gallium‑68 or F‑18 tracers). This scan not only confirms the presence of prostate cancer, but more importantly, it shows whether the cancer cells are expressing PSMA strongly enough to be targeted.
If the scan lights up—meaning the tumors are PSMA-positive—the patient is considered a suitable candidate for this therapy. Additional tests like blood counts, kidney function, and liver function help determine the patient’s readiness and safety profile for the treatment.
A Targeted Revolution in Prostate Cancer Care
For many men around the world, the diagnosis of metastatic or hormone-resistant prostate cancer is a turning point—often filled with fear, uncertainty, and questions. What if chemo doesn’t work anymore? What if hormone therapy has failed?
That’s where Lu‑177 PSMA Therapy for Hormone-Resistant Prostate Cancer in Germany steps in—as a scientifically advanced and emotionally reassuring path forward. It offers not just another treatment, but hope grounded in data, compassion, and expertise.

Global Approvals
- FDA Approved: March 2022, for PSMA-positive metastatic castration-resistant prostate cancer (mCRPC)
- EMA Approved: December 2022, following the success of the VISION trial
This makes Germany not just a leader in innovation but also one of the safest and most reliable destinations for Lu‑177 PSMA therapy under global treatment protocols.
Why Germany for International Patients?
Germany was one of the first countries to develop and offer Lu‑177 PSMA therapy, even before FDA/EMA approval, under compassionate-use frameworks. Today, it stands as a hub for international cancer patients, offering:
- Advanced treatment options
- Streamlined medical visa support
- High-quality care with transparent cost structure
- Flexible scheduling for those traveling from outside Europe
- Well-experienced doctors and medical staff
- Hospitals equipped with latest equipments and medical technologies
Hospitals in cities like Berlin, Munich, Heidelberg, Marburg, and Cologne are well-equipped with English-speaking teams and coordinated cancer boards. Cancer Rounds provides complete end-to-end assistance for international patients seeking Lu-177 PSMA therapy for prostate cancer treatment in Germany.
Essential Qualities of Doctors for Lutetium‑177 PSMA Therapy
Doctors qualified to administer Lu‑177 PSMA therapy must possess a unique blend of clinical, technical, and interdisciplinary expertise. These specialists are typically nuclear medicine physicians or radiation oncologists with advanced training in radioligand therapies and prostate cancer management. Key qualities include:
- Board certification in nuclear medicine or oncology
- Expertise in PSMA-targeted imaging and therapies (e.g., PSMA PET-CT interpretation)
- Experience in handling radioisotopes, particularly Lutetium-177
- Strong understanding of prostate cancer staging, progression, and hormone resistance
- Skills in personalizing treatment plans based on tumor burden, prior therapies, and patient fitness
- Close collaboration with urologists, medical oncologists, and radiologists
- Facility with monitoring toxicity and managing side effects such as xerostomia, fatigue, and marrow suppression
- Ongoing involvement in clinical research or trials to stay updated on evolving PSMA therapies
Top-tier doctors also ensure patient education, empathy, and transparency throughout the treatment journey. Their goal is not only to target resistant cancer cells but also to maximize quality of life and long-term outcomes.
Top Hospitals in Germany Offering Lutetium-177 PSMA Therapy
| Hospital Name | Specialty |
|---|---|
| Helios Hospital Berlin-Buch | One of Europe’s largest nuclear medicine and oncology centers |
| University Hospital Heidelberg – National Center for Tumor Diseases (NCT) | Birthplace of PSMA therapy; pioneers in Lu‑177 development |
| University Hospital Rechts der Isar – Technical University of Munich (TUM) | Advanced nuclear medicine and personalized oncology |
| University Hospital Marburg (UKGM) | High-volume center for Lu‑177 PSMA therapy and trials |
Leading Nuclear Medicine Experts for Lutetium‑177 PSMA Therapy in Germany
The top doctors in Germany for Lu-177 PSMA therapy are as follows:
| Name | Specialization | Experience | Publications | Notable Expertise |
| Prof. Dr. med. Winfried Brenner | Nuclear Medicine | 32 years | 582 | Advanced radioligand therapy and PSMA-targeted theranostics |
| Prof. Dr. med. Wolfgang Weber | Nuclear Medicine, Molecular & Radionuclide Therapy | 30 years | 2,474 | Global leader in radionuclide therapy and personalized oncology |
| Prof. Dr. med. Hans-Jürgen Biersack | Nuclear Medicine | 53 years | 548 | Pioneer in theranostics and targeted radiopharmaceuticals |
| Prof. Dr. med. Frank Grunwald | Nuclear Medicine | 41 years | 524 | Expert in clinical nuclear oncology and patient-tailored therapy |
| Prof. Dr. med. Stefan Dresel | Nuclear Medicine, Radiology, Radiation Therapy, Theranostics | 34 years | 149 | Known for combining imaging and therapy in prostate cancer care |
| Prof. Dr. med. Michael Schäfers | Nuclear Medicine | 31 years | 399 | Specialist in hybrid imaging and PSMA-labeled tracers |
| Prof. Dr. med. Samer Ezziddin | Nuclear Medicine | 28 years | 252 | Authority in Lu‑177 PSMA therapy with extensive clinical trials |
| Prof. Dr. med. Uwe Haberkorn | Nuclear Medicine | 37 years | 561 | Renowned for translational research and PSMA PET-based therapies |
| Prof. Dr. med. Ambros J. Beer | Nuclear Medicine | 26 years | 410 | Focused on precision oncology and radiopharmaceutical innovation |
Indicative Cost of Lutetium‑177 PSMA Therapy in Germany
The cost of Lu‑177 PSMA Therapy in Germany typically ranges between €9,000 to €22,500 per cycle, depending on the medical center’s infrastructure, diagnostic protocols, and support services. A complete course of treatment—usually consisting of 3 to 4 therapy cycles—may cost between €28,000 and €60,000. This estimate generally includes essential diagnostics such as PSMA PET-CT scans, laboratory tests, inpatient hospital care, medical supervision, and post-therapy follow-ups. Many hospitals also offer bundled service packages that include interpreter assistance, local accommodation, and logistical support for international patients.
Cost of Lutetium‑177 PSMA Therapy in Germany (City-wise Range)
- Lutetium‑177 PSMA Therapy in Berlin, Marburg, Cologne: €9,000–14,000 per cycle
- Lutetium‑177 PSMA Therapy in Munich, Heidelberg: €14,000–22,500 per cycle (more advanced diagnostics and accommodation)
- Total estimated cost for full treatment (3–4 cycles): €28,000–€60,000, including PSMA PET-CT, labs, hospital stay, and follow-ups
Patients can often bundle accommodation, interpreter, and transportation into complete care packages.
Cancer Rounds has dedicated case managers to provide you complete financial guidance for Lu-177 PSMA treatment in Germany. The team also provides assistance for visa and travel process, accommodation, prior virtual consultation, and in-person consultation and treatment in Germany.
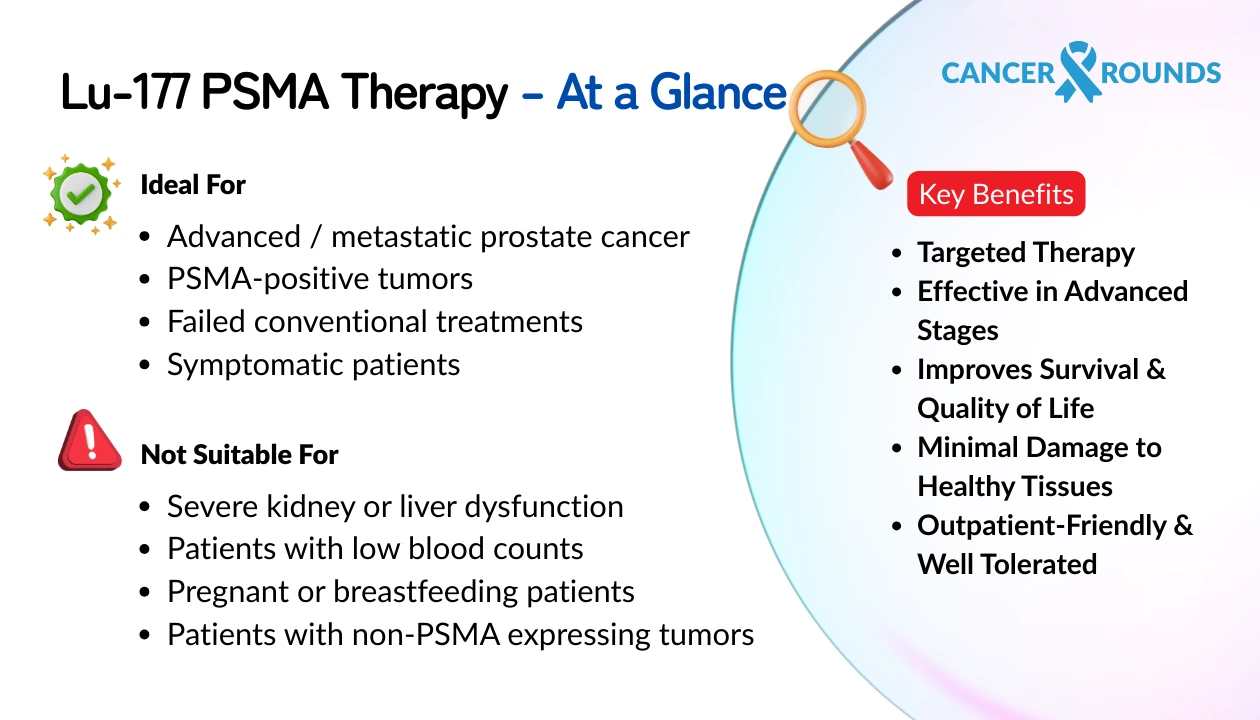
Indications & Ideal Patient Profile
Lu‑177 PSMA Therapy in Germany is primarily recommended for:
- Metastatic prostate cancer not responding to hormone therapy
- Patients with high PSMA expression on PET/CT
- Those with good bone marrow, kidney, and liver function
- International patients with documented treatment failure after chemotherapy
It is now also being explored for early-stage prostate cancer in high-risk patients, as shown in clinical trials assessing Lu‑177 PSMA Therapy role in early prostate cancer cases in Germany.
Contraindications
- Poor kidney function
- Inadequate bone marrow reserve
- PSMA-negative tumors on PET/CT
- Severely debilitated patients with multiple uncontrolled comorbidities
Lu‑177 PSMA Therapy vs Chemotherapy for Prostate Cancer in Germany
| Feature | Lu‑177 PSMA Therapy | Chemotherapy |
| Mechanism | Targeted radioligand therapy | Systemic cytotoxic agents |
| Side Effects | Dry mouth, fatigue, mild nausea | Hair loss, infections, fatigue |
| Tolerance | High | Moderate to poor |
| Response Rate | 45–80% | ~30–40% |
| Hospital Stay (Germany) | 3–4 days | Outpatient or admission-based |
| International Accessibility | Yes – streamlined in Germany | Varies by country |
Conclusion: For many patients, Lu‑177 offers a more precise, better-tolerated, and longer-lasting response than traditional chemotherapy.
Success Rates & Clinical Outcomes
- Biochemical response (PSA drop): ~80%
- Tumor shrinkage on scans: 45–60%
- Median progression-free survival: ~8.7 months
- Median overall survival: ~15.3 months
- Patient-reported quality of life: Significantly improved
These results make it an excellent option for patients who have exhausted traditional lines of care.
Why Patients from the UK, USA, and Worldwide Choose Germany for Advanced Cancer Treatment
For international patients seeking the most advanced, precision-targeted treatment for metastatic castration-resistant prostate cancer (mCRPC) abroad, Lu-177 PSMA Therapy in Germany represents a revolutionary option. Germany’s leadership in nuclear medicine, radioligand therapy, and advanced prostate cancer care attracts individuals from the UK, US, Canada, Ireland, Saudi Arabia, Holland, the broader Middle East, and worldwide. If you’re an international patient navigating highly specialized medical landscapes, Cancer Rounds serves as your dedicated radiopharmaceutical therapy facilitator, prostate cancer expert, and international patient coordinator, simplifying access to Germany’s world-class Lu-177 PSMA therapy clinics and specialized services in leading nuclear medicine centers in cities like Heidelberg, Essen, Munich, and Berlin.
Why International Patients Prioritize Germany for Lu-177 PSMA Therapy
German medical institutions are globally recognized for their cutting-edge approach to nuclear medicine and prostate cancer, seamlessly integrating advanced diagnostics (PSMA PET/CT), specialized radiopharmaceutical production, precise therapy administration, and rigorous patient monitoring. Patients choose Germany for compelling reasons when considering Lu-177 PSMA Therapy medical tourism:
- Pioneering Radioligand Therapy Expertise: Germany has been at the forefront of developing and implementing Lu-177 PSMA therapy, with extensive clinical experience. Leading nuclear medicine centers, particularly in Heidelberg and Essen, offer proven protocols and specialized teams for this highly effective precision radiation therapy for prostate cancer.
- Integrated PSMA PET/CT Diagnostics: Accurate patient selection is crucial. German facilities excel in advanced PSMA PET/CT imaging, which precisely identifies prostate cancer cells expressing the PSMA protein, ensuring that the Lu-177 PSMA therapy is targeted specifically to active disease. This advanced diagnostic capability is vital for the success of PSMA-targeted therapy in Germany.
- Specialized Nuclear Medicine Infrastructure: Administering Lu-177 PSMA requires dedicated facilities and highly trained personnel. German hospitals have the sophisticated infrastructure, licensed radiopharmacies, and experienced nuclear medicine specialists to safely and effectively deliver this complex treatment, making them leaders in radiopharmaceutical cancer treatment in Europe.
- Comprehensive Prostate Cancer Care Integration: Lu-177 PSMA therapy is often integrated into a broader management strategy for mCRPC. German specialists, working in centers across Munich and Berlin, are skilled in combining this novel treatment with other systemic therapies to optimize outcomes and manage advanced disease effectively.
- Access to Advanced Prostate Cancer Trials: Germany is actively involved in ongoing research to further refine and expand radioligand therapies. This means international patients may gain access to clinical trials for next-generation radiopharmaceuticals or combination therapies, offering hope for those seeking cutting-edge prostate cancer treatments in Europe.
Cancer Rounds: Your Trusted Partner for Accessing Lu-177 PSMA Therapy in Germany
At Cancer Rounds, we deeply understand the unique complexities involved in accessing highly specialized, innovative treatments like Lu-177 PSMA Therapy – from identifying the right nuclear medicine centers and navigating stringent eligibility criteria to managing logistics for radiopharmaceutical care. We act as your essential conduit, directly connecting you with Germany’s top Lu-177 PSMA therapy clinics and leading nuclear medicine specialists in key academic hubs like Heidelberg, Essen, Munich, and Berlin. We are experts in international patient services for advanced prostate cancer therapies.
For Patients from the UK Seeking Lu-177 PSMA Therapy
If you are from the UK exploring advanced, targeted therapies for mCRPC or require an expert assessment for Lu-177 PSMA therapy beyond what’s available through the NHS, Cancer Rounds provides direct pathways to German innovation. We facilitate prompt consultations and access to leading nuclear medicine departments, ensuring you receive comprehensive medical review from esteemed overseas specialists. This is for UK prostate cancer patients seeking treatment abroad.
For Patients from the US Seeking Radioligand Therapy Expertise
Patients from the US looking for pioneering European radioligand therapy approaches, access to established Lu-177 PSMA protocols, or a second opinion from German nuclear medicine experts can leverage Cancer Rounds’ services. We offer expedited appointments and connections to leading specialists in cities like Essen and Munich, simplifying your journey to obtain advanced, personalized prostate cancer care in Germany. This is for US prostate cancer patients considering international treatment.
For Patients from the Middle East and Saudi Arabia Considering Advanced Prostate Cancer Treatments
We recognize the unique considerations for patients from the Middle East and Saudi Arabia when pursuing cutting-edge Lu-177 PSMA therapy in Europe. Cancer Rounds meticulously streamlines your journey, arranging swift access to leading German nuclear medicine specialists in cities such as Heidelberg and Berlin, facilitating expert consultations for therapy suitability, and linking you with this precise radioligand treatment. We are your dedicated ally in securing high-quality innovative prostate cancer care in Germany without the typical logistical complexities.
For Patients from Canada, Ireland, Holland, and Other International Regions
Whether you are from Canada facing specific access challenges for Lu-177 PSMA therapy, or from Ireland, Holland, and other international locations seeking world-class radioligand treatment for prostate cancer, Cancer Rounds ensures a seamless and efficient process. We simplify access to specialized Lu-177 PSMA programs and personalized treatment planning in Germany’s leading nuclear medicine centers, empowering you to focus entirely on your health and potential recovery.
Our Comprehensive Support for Your Specialized Medical Journey
Cancer Rounds offers meticulous, end-to-end support tailored for patients accessing highly specialized treatments like Lu-177 PSMA Therapy. Our services encompass all logistical intricacies, including medical record translation, visa assistance, travel arrangements, specialized accommodation, and crucial language assistance throughout your medical journey in Germany. We meticulously manage these details so you can concentrate solely on your health and treatment, making international medical travel for radioligand therapy stress-free. Our extensive network guarantees you receive a bespoke treatment strategy, leveraging Germany’s advanced medical capabilities, precisely tailored to your individual needs as an international prostate cancer patient.
Do not let geographical distance or administrative complexities impede your access to pioneering Lu-177 PSMA Therapy in Germany. Let Cancer Rounds be your trusted guide to receiving timely, comprehensive, and advanced prostate cancer care in Germany’s celebrated nuclear medicine centers. Contact us for Germany cancer treatment cost information and to understand more about how to get innovative treatment in Germany for cancer.
International Patient Success Stories
- Patient from India: After failing chemotherapy, Lu‑177 in Marburg brought his PSA from 210 to 15 in 3 months.
- Patient from the UK: Stage 4 diagnosis, one year remission post-therapy in Munich.
- Patient from UAE: Stable disease for 11 months with good energy levels after treatment in Cologne.
- Patient from Brazil: Had one PET-negative lesion but was still treated successfully under close board supervision in Heidelberg.
FAQ – Lutetium‑177 PSMA in Germany
1. What is Lutetium‑177 PSMA therapy?
Lu‑177 PSMA therapy is a targeted radionuclide treatment for advanced prostate cancer. It uses a radioactive isotope called Lutetium-177 attached to a molecule that binds specifically to PSMA (Prostate-Specific Membrane Antigen) found on cancer cells, delivering focused radiation to destroy them while sparing healthy tissue.
2. Who is eligible for Lu‑177 PSMA therapy in Germany?
Lu‑177 PSMA therapy treatment is typically offered to patients with metastatic castration-resistant prostate cancer (mCRPC) who have progressed despite hormone therapy or chemotherapy. Eligibility is confirmed via PSMA PET-CT scan to ensure PSMA expression on cancer cells.
3. What are the benefits of receiving Lu‑177 PSMA therapy in Germany?
Germany offers world-class nuclear medicine centers, state-of-the-art imaging, experienced doctors, and access to personalized radioligand therapies. Many hospitals are involved in clinical trials and offer care that meets international standards.
4. What is the cost of Lu‑177 PSMA therapy in Germany?
The average Lutetium‑177 PSMA Therapy cost per cycle ranges from €9,000 to €22,500, depending on the hospital and services. A complete course of 3–4 cycles typically costs €28,000–€60,000, inclusive of PSMA PET-CT scans, labs, hospital stay, follow-up care, and supportive services like interpretation or accommodation.
5. How is the therapy administered?
Lu‑177 PSMA is given via intravenous injection in a controlled hospital environment. The procedure takes around 30–60 minutes. Patients may stay in the clinic for a few hours or overnight to monitor for side effects.
6. What hospitals offer Lu‑177 PSMA therapy in Germany?
Top hospitals in Berlin, Munich, Heidelberg, Cologne, and Marburg offer this therapy, including academic centers and certified nuclear medicine departments with experience in PSMA-targeted radionuclide therapy.
7. Are there side effects to Lu‑177 PSMA therapy?
Most side effects are mild, such as fatigue, dry mouth (xerostomia), nausea, and temporary low blood counts. Severe complications are rare but are monitored by the clinical team.
8. How many cycles of Lu‑177 PSMA are usually needed?
Most patients receive 3 to 6 treatment cycles, spaced 6 to 8 weeks apart, depending on response and tolerance. Doctors may adjust the schedule based on PSA levels, imaging results, and clinical symptoms.
9. How effective is Lu‑177 PSMA treatment in Germany?
Clinical studies from leading German centers show PSA reduction in over 70% of patients. Many experience improved quality of life, pain control, and disease stabilization, especially in late-stage prostate cancer.
10. Is Lu‑177 PSMA therapy covered by insurance?
In Germany, private insurance may cover the therapy partially or fully. For international patients, treatment is usually self-funded, but hospitals often provide cost estimates and payment packages in advance.
11. Do I need a PSMA PET-CT scan before therapy?
Yes. A PSMA PET-CT scan is mandatory to confirm the presence of PSMA-positive cancer cells. Without PSMA uptake, the therapy would not be effective or safe.
12. Can international patients access Lu‑177 PSMA in Germany?
Absolutely. Germany welcomes medical tourists for prostate cancer treatment. Most hospitals have international departments to help with visas, travel, interpreter services, and tailored treatment planning.
13. What makes German nuclear medicine centers special for Lu‑177 PSMA?
German hospitals offer integrated theranostics (therapy + diagnostics), highly trained nuclear medicine physicians, and access to research-based protocols, making them leaders in precision oncology.
14. Can I continue other medications during Lu‑177 therapy?
Doctors will evaluate your case, but many supportive or chronic condition medications can continue. Hormone therapy may also be combined if clinically indicated.
15. What happens after the treatment is complete?
Post-treatment follow-up includes regular blood tests, PSA monitoring, and imaging (like PET or MRI) every few months. Doctors also provide supportive care and guidance for managing any long-term effects.
You May Be Also Interested In
All Treatment Pages
Related Patient Stories

I was battling prostate cancer and back pain when I found Cancer Rounds. They were incredibly helpful, handling everything from the visa to the treatment and hotel stay. I am truly grateful for their support.

Thanks to Cancer Rounds, I got timely prostate cancer care in India. From fast visa help to expert treatment, everything was handled smoothly. My PSA dropped from 254 to 1, & I’m truly grateful.

I came from Cameroon with Cancer Rounds’ help. Thanks to Dr. Rajat Bajaj at Fortis, I’m walking again and feeling much better.
Our Impact
CancerRounds is making quality cancer care accessible to more people every day.




Why Choose India for Cancer Treatment?

World-Class Care
Skilled oncologists provide top-tier medical services

Affordable Treatment
Costs are significantly lower than in Western countries.

Comprehensive Packages
Hospitals offer all-inclusive plans covering surgery, stay, and aftercare.
Easy Accessibility
Well-connected airports and international flight routes.

Proven Success
High patient satisfaction and positive treatment outcomes
Thank You!
Your form has been submitted successfully.